Abstract
Introduction: Enhancer of zeste homolog (Ezh)-2 is a critical component of polycomb repressive complex, essential for self-renewal and differentiation of hematopoietic stem cells. EZH2 (chromosome 7q) mutations reported in myeloid disorders are loss of function type, with an incidence of 4-6% in published literature. Presence of EZH2 mutation has been linked to poor overall survival (OS) in patients (pts) with myelodysplastic syndromes (MDS) in studies (Bejar et al. 2015). However, potential clinical and genomic determinants of outcome in EZH2- mutant (MT) MDS is not well characterized.
Methods: In this large retrospective study, all pts treated for MDS at Moffitt Cancer Center with available next generation sequencing (NGS) results at diagnosis were eligible for inclusion. Clinical, demographic, and genomic data were collected from the annotated institutional MDS database. Patients in whom the NGS panel did not include EZH2 and those with benign or variants of unknown significance were excluded from the analyses. Survival estimates using Kaplan-Meier statistics and multivariate analysis with Cox regression were performed in SPSS (v.26).
Results: Our study included a total of 1,774 pts with MDS, with 83 (4.7%) harboring a pathogenic EZH2 mutation [Table 1]. Patients with EZH2-MT MDS were older than EZH2-wild type (EZH2-WT) group (median age at diagnosis- 72 vs. 69 years, p= 0.010). No gender difference was noted between groups. Both groups had similar baseline hemoglobin, white blood cell, platelet, and bone marrow blast counts. Complex karyotype was less frequent in EZH2-MT pts compared to their WT counterparts (7% vs. 18%, p= 0.013). Concurrent deletion (7q) or monosomy 7 was noted in 12% pts with EZH2-MT MDS. Myelodysplastic syndrome with excess blasts was the most common WHO 2016 classification subtype in both EZH2-MT and WT pts. Approximately 36% pts in EZH2-MT and 38% in EZH2- WT groups fell into high or very high prognostic risk categories by revised International Prognostic Scoring System (IPSS-R).
The most common co-occurring somatic mutation in EZH2-MT pts was ASXL1, with a significantly higher frequency than EZH2-WT (54% vs. 19%, p = <0.001). Mutations in TET2, RUNX1, CBL, and ZRSR2 were also significantly more prevalent in EZH2-MT group compared to EZH2-WT; SRSF2 and TP53 mutations were relatively less common in EZH2-MT pts.
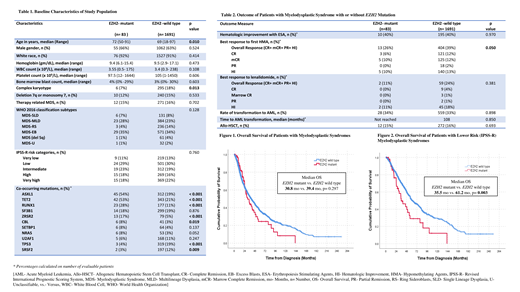
Treatment with erythropoiesis stimulating agents led to a hematologic improvement in 40% pts in both groups [Table 2]. Patients with EZH2-MT MDS showed a trend for lower rates of overall response (OR) and complete remission (CR) to hypomethylating agents (HMA) compared to those with EZH2-WT MDS (26% vs. 39%, and 6% vs. 12%, respectively; p= 0.050). Response to treatment with lenalidomide was similar in both groups.
There was no significant difference in the rate of progression to AML or time to AML transformation between groups. Median overall survival (mOS) was 30.8 months in pts with EZH2-MT MDS, compared to 39.4 months in EZH2-WT group (p= 0.297) [Figure 1]. In pts with IPSS-R lower risk (LR- very low, low, and intermediate) MDS, presence of EZH2 mutation was associated with inferior survival (mOS- 35.5 vs. 61.2 months, p= 0.003) [Figure 2]; however, it was not an independent predictor of OS in LR pts after adjustment for age at diagnosis, ASXL1 and RUNX1 mutational status in MVA. In the EZH2-MT group, pts with EZH2-MT ASXL1-WT RUNX1-WT MDS (n=29) had a significantly prolonged mOS compared to EZH2-MT ASXL1-MT/ RUNX1-MT pts (48.7 vs. 26.8 months, p- 0.031). Concurrent chromosome 7 abnormalities were associated with significantly worse OS (mOS- 20.8 vs. 35.5 months, p= 0.002) among EZH2-MT pts.
Conclusions: In our study, the incidence of pathogenic EZH2 mutation was 4.6%, comparable to prior reports. Patients with EZH2-MT MDS were significantly older, more commonly harbored deleterious ASXL1 and RUNX1 mutations, and showed a trend towards lower CR and OR rates to HMA. Median OS did not differ overall between EZH2-MT and WT groups. Presence of an EZH2 mutation was a predictor of OS in IPSS-R LR pts, although it was not independent on MVA adjusting for age, ASXL1, and RUNX1 mutational status. In the EZH2-MT group, concurrent del (7q) or monosomy 7 was associated with worse OS, indicating biallelic loss of function. Future clinical trials should explore potential role of novel targeted therapies in improving outcome in pts with EZH2-MT MDS.
Hussaini: Stemeline Therapeutics: Honoraria. Tinsley-Vance: Jazz: Consultancy, Speakers Bureau; Taiho: Consultancy; Abbvie: Honoraria; Astellas: Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy; Fresenius Kabi: Consultancy. Kuykendall: PharmaEssentia: Honoraria; Novartis: Honoraria, Speakers Bureau; Incyte: Consultancy; Prelude: Research Funding; CTI Biopharma: Honoraria; Celgene/BMS: Honoraria, Speakers Bureau; BluePrint Medicines: Honoraria, Speakers Bureau; Abbvie: Honoraria; Protagonist: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sweet: AROG: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet: AbbVie: Consultancy; Millenium Pharma/Takeda: Consultancy; Daiichi Sankyo: Consultancy; BerGenBio: Consultancy; ElevateBio Management: Consultancy; Astellas: Consultancy; Agios: Consultancy; Celgene/BMS: Consultancy; Jazz: Consultancy. Sallman: Takeda: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellia: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Magenta: Consultancy. Padron: Blueprint: Honoraria; Taiho: Honoraria; Incyte: Research Funding; BMS: Research Funding; Stemline: Honoraria; Kura: Research Funding. Komrokji: Taiho Oncology: Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; AbbVie: Consultancy; Acceleron: Consultancy; Jazz: Consultancy, Speakers Bureau; BMSCelgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.